The Special Color Effect in “Chameleon” Amber


One special variety of Burmese amber is “chameleon” amber, named for the bluish green color that appears to float on its surface (figure 1). This material is found in the famous Tengchong market in Yunnan Province, China’s largest Burmese amber market. Its bodycolor ranges from golden brown to brownish red or even red (figure 2A). When exposed to sunlight or strong white light against a black background, its surface shows a uniform green color (figure 2B). Chameleon amber is rare and expensive compared to regular Burmese golden or brownish amber, especially with the severe reduction of exports from Myanmar.
Until recently, few studies have been conducted on chameleon amber. X. Jiang et al. speculated that the green surface color might be caused by the superposition of its bodycolor and the fluorescence of amber (“Gemmological and spectroscopic characteristics of different varieties of amber from the Hukawng Valley, Myanmar,” Journal of Gemmology, Vol. 37, No. 2, 2020, pp. 144–162). C.C. Shuai et al. proposed that the “light retention” effect was related to elevated sulfur and calcium contents (“The spectrum characteristic research of color change effect and ‘light tarry effect’ amber from Burma,” Spectroscopy and Spectral Analysis, Vol. 40, 2020, pp. 1174–1178). Z. Shi et al. reported that the effect might be due to unique internal aromatic hydrocarbon structures (“Spectral characteristics of unique species of Burmese amber,” Minerals, Vol. 13, No. 2, 2023, article no. 151).
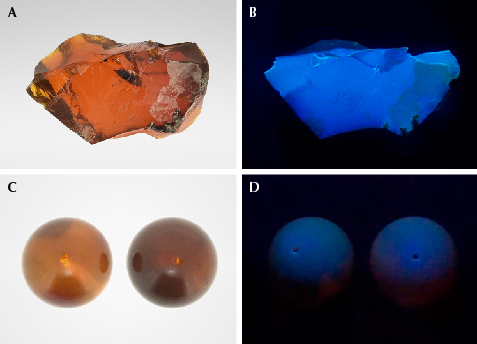
For this study, three Burmese chameleon amber specimens were acquired from the Tengchong market (see figures 1, 2A, and 3A). All three displayed a translucent brownish red bodycolor, with a few bubbles and flow lines within them. The bluish green floating color was observed when the specimens were rotated. This phenomenon is most obvious with a black background. Standard gemological properties and Fourier-transform infrared spectroscopy data were consistent with those of regular Burmese amber. While the bluish green floating color might be related to fluorescence, no such effect is seen on other types of fluorescing Burmese amber (e.g., brownish amber). To further explore this, brownish Burmese amber beads with bodycolor similar to that of chameleon amber were selected as a reference (figures 2C and 3C). Their bodycolor was not as vivid as chameleon amber, and it did not display the bluish green floating fluorescence. Observation under a UV lamp showed that the chameleon amber in figure 3A produced strong bluish white fluorescence (figure 3B) in long-wave UV (365 nm), while the brownish amber beads in figure 3C showed a weak bluish purple fluorescence (figure 3D).

The samples were then examined with 3D fluorescence spectroscopy (figure 4) under the excitation of a continuous-wavelength excitation light source. The fluorescence intensity of brownish amber was clearly much lower than that of chameleon amber. In addition, two peaks (455 and 384 nm) were absent in the excitation spectrum of brownish amber. A high degree of similarity was exhibited in the samples’ emission spectra, with all the peaks positioned at approximately 434, 464, 469, and 497 nm. However, the biggest difference was in the fluorescence intensity, which was easily observed in chameleon amber.
The 3D fluorescence spectra of chameleon amber exhibited several fluorescence centers, mainly at 434, 464, 469, and 497 nm generated by excitation wavelengths of 386, 406, 436, and 457 nm. The fluorescence color could be calculated by the fluorescence centers, and the results showed that the spectral color was blue under 386 nm excitation and bluish green under 436 nm excitation. We believe that the bluish green floating color under sunlight is caused by the bluish green fluorescence center at 469 nm under 436 nm excitation. This is much more consistent with observations in daylight. Due to the low fluorescence intensity of brownish amber, its blue fluorescence color is very weak, and most light penetrates it without producing any special optical effect. The difference in fluorescence intensity between the 469 and 434 nm fluorescence centers corresponds with the relative spectral power distribution of the light source. Under the excitation of a xenon lamp, the fluorescence center at 434 nm is more intense than the light-emitting center at 469 nm. However, amber is usually observed in daylight. The optimal excitation wavelengths for the emission centers at 434 and 469 nm are 386 nm and 436 nm, respectively. From the relative spectral power distribution of the two light sources, it can be seen that the spectral density of the xenon lamp at 386 nm is greater than 436 nm, while the D65 daylight-equivalent source is the opposite. Against a dark background and under daylight, the fluorescence of chameleon amber should be dominated by the bluish green fluorescence emitted at 436 nm.
The fluorescence center at 469 nm is very common in Baltic, Dominican, Mexican, and Burmese amber. This fluorescence center in chameleon amber is unique for its high intensity and separation from other fluorescence colors. Only if the fluorescence intensity is high enough will it show as the dominant fluorescence color. The optimal excitation wavelength (436 nm) corresponding to the 469 nm fluorescence center is far removed from the optimal excitation wavelength of other strong fluorescence centers; therefore, the fluorescence color is not a mixture of multiple colors. Many varieties of amber can also produce strong fluorescence at 469 nm, but often mixed with other fluorescence colors of similar intensity. For example, the fluorescence peaks of 448 and 474 nm in Dominican blue amber always appear together, and their strong fluorescence peaks can be seen with exposure to the same excitation wavelength. In most cases, the fluorescence intensity of the former is slightly greater, which leads to the fluorescence of the blue amber being mainly blue, without a green modifier. Some Dominican blue amber with uneven fluorescence distribution can also show a mosaic pattern (X. Chenxing et al., “Characterisation of patchy blue and green colouration in Dominican blue amber,” Journal of Gemmology, Vol. 37, No. 7, 2021, pp. 700–713). The fluorescence distribution of the chameleon amber tested in this study was uniform, with high intensity and good separation, producing a high-quality bluish green floating fluorescence effect.



